GOST 1367.7-83
Group B59
INTERSTATE STANDARD
ANTIMONY Methods of determining sulphur
GOST 1367.7−83 Cherries GOST 1367.7−76
Antimony. Methods Gogh the determination of sulphur
OXGW 1709
The decision of the State Committee of the USSR, but the standards of 16 December 1983 Ls 6013 date of introduction installed 0I.01.8 S
Limitation of actions taken under the Protocol .Ne 4−93 of the Interstate Council for standartami. Metrology and certification (ICS 4−94)
This standard sets the polarographic method for the determination of sulfur from 5−10 s to MO-3 %, and volumetric method for the determination of sulfur from 0.005 to 0.2% antimony.
1. GENERAL REQUIREMENTS
1.1. General requirements for methods of analysis and security requirements — according to GOST 1367.0−83.
2. POLAROGRAPHIC METHOD
The method is based on extracting and recovering sulfur from metallic antimony with a mixture of itestosterone and hydrochloric acids with sodium hypophosphite with the subsequent Stripping of hydrogen sulfide in a current of inert gas. The content of the sulfide ion is determined polarographically on the AC polarography.
2.1. Apparatus, reagents and solutions
Polarograph type PU-1 with mercury dripping electrode, or any other polarograph AC.
The electrolyzer with a remote anode. In the anode compartment h&Titus mercury. The anode compartment and every day is filled with saturated solution of potassium chloride.
Volumetric flasks according to GOST 1770−74 capacity of 100. 200 cm3.
Flask, glass, laboratory for GOST 25336−82 with a capacity of 2 DM3.
Refrigerators are glass laboratory according to GOST 23932−90.
Pipettes with graduation marks N GD a capacity of 1, 2, 5 and 10 cm5.
The unit at the household GOST 14919−83.
Mercury metal according to GOST 4658−73, grade 1*0.
Water bidistillate. is prepared by distillation of distilled water in a quartz distillation apparatus.
Potassium chloride according to GOST 4234−77, OS. h, a saturated solution.
Hydrochloric acid, OS. h, GOST 14261−77 and 6 mol/DM3 solution.
Sulfuric acid according to GOST 4204−77, 0.05 mol/DM3 solution (fixanal).
For nitric acid GOST 4461−77.
Hydrofluoric acid according to GOST 10484−78.
For acetic acid GOST 61−75.
Itestosterone acid according to GOST 4200−77.
Sodium posterolaterally (hipofosfit sodium) according to GOST 200−76.
Official edition Reprinting is prohibited
Edition with Change number I. approved in March 1989 (IUS 6−89).
49
C. 2 GOST 1367.7−83
The mixture recovery: in a flask with a capacity of 2 DM3, equipped with a reflux condenser, hurt 150 grams of hypophosphite sodium, flow of 300 cm3 of concentrated hydrochloric acid, 500 cm5 itestosterone acid. 200 cm' with bidistilled water, stirred and boiled in a nitrogen flow of 7−8 h to remove sulfur. After cooling the solution is decanted from the salts and stored in a flask made of dark glass with a glass stopper.
Potassium hydroxide according to GOST 24363−80. 2 mol/DM3 and a solution with a mass fraction of 25%. 2 mol/DM3 solution of potassium hydroxide is prepared in svezhekipyachenoy the double-distilled water for 2−3 days before use.
Pyrogallol on the other 6−09−5319−86. a solution with a mass fraction of 25% in a solution with a mass fraction of 25% potassium hydroxide.
The gaseous nitrogen according to GOST 9293−74. Before entering the distillation apparatus for nitrogen, purified passing it through two flasks Drexel with a solution of pyrogallol with a mass fraction of 25% in a solution with a mass fraction of 25% potassium hydroxide.
Sodium sulfide (sodium sulfide) according GOST 2053−77.
Ammonia water according to GOST 3760−79.
Disodium salt ethylendiaminetetraacetic acid (Trilon B) according to GOST 10652−73: 500 g Trilon B was placed in a beaker with a capacity of 1 DM5, poured 700 cm5 water and, while stirring, pour the ammonia to dissolve the precipitate. The solution is filtered through a dense filter, poured 6 mol/DM3 hydrochloric acid solution to neutralize the solution (on the paper of the Congo) and an excess of 100 cm3. Filter the solution through a Buchner funnel. Precipitate was washed 10 times with double-distilled water and air dried.
The hydroxyls" hydrochloride according to GOST 5456−79. 2 mol/DM3 solution is prepared in souprecipe-cluded in double-distilled water a day application.
Background polarographic: 80 cm5 2 mol/DM' of solution of potassium hydroxide containing 10 g Trilon B in 100 cm' of solution, pour 20 cm3 of 2 mol/DM5 solution hydroxylamine hydrochloride and 150 cm3 svejeprokipachenna double-distilled water. The solutions are mixed the day of application.
Standard solutions of sulfur
The solution, As sulfate sulfur: 6,25 cm3 0.05 mol/DM3 sulfuric acid solution, prepared of fiksanala, poured into a measuring flask with a capacity of 100 cm3, and top up with double-distilled water to the mark and mix.
I see' solution, And sulphate sulphur contains 0.1 mg
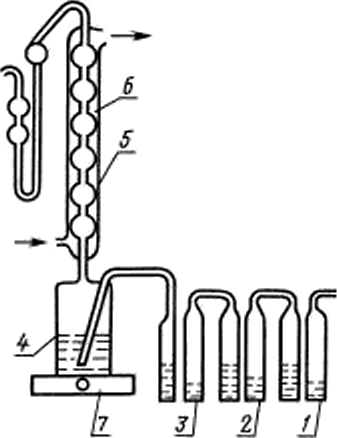 |
/, 2 — bubblers. filled with 25% solution of pyrogallol in 2S %of the races lore g of potassium hydroxide: 3 — barbers билис1илдя-4 — flask reaction machinegu 200 cm3 connected to the refrigerator with mosio sockets: 5 — refrigerator length 25 cm: 6 receiver machinegu 20 cm 7 — -5Лектрог1ЛИ1ка. Barbary and receiver are connected by a vinyl chloride grebkami |
sulfur.
The solution of sulphate sulphur: 1 cm3 solution, And transferred to a volumetric flask with a capacity of 100 cm3 I drink to the mark with double-distilled water and stirred; prepared on the day of application.
1 cm of a solution of sulphate sulphur contains 1 mcgeary. Solution, And sulfide sulfur: 0.15 g of dried filter paper sodium sulphide containing 20 mg of sulfur, placed in a volumetric flask with a capacity of 100 cm3, pour 20 cm3 of the polarographic background, top up with bidistillated to the mark and mix; prepared on the day of application.
1 cm3 of solution, And sulfide sulfur contains 0.2 mg
sulfur.
A solution of sulfide sulfur: 1 cm3 solution, And transferred to a volumetric flask with a capacity of 200 cm3, made up of the polarographic background to the mark and mix; cook before eating.
1 cmj of a solution of sulfide sulfur contains I mcg
sulfur.
Setup for the determination of sulfur (hell. 1). (Changed edition, Rev. No. 1).
2.2. Preparation for assay
1.
Damn. I
50
GOST 1367.7−83 C. 3
Noah, hydrofluoric, and acetic acids taken at the ratio of 25:15:25, 30 C. Filtered through a Buchner funnel and washed with plenty of water and then with double-distilled water. The sample was dried in air and crushed in a Jasper or an agate mortar into a powder with a particle size less than 0.074 mm on the day of analysis.
(Changed edition, Rev. No. 1).
2.2.2. Starting the analysis, washed the refrigerator installation with double-distilled water, as when the device is in working condition on the inner surface of the tube of the refrigerator may instiutional acid oxidation by atmospheric oxygen, which can lead to an underestimation of the results of the analysis.
2.2.3. Correctness of installation check a standard Stripping solution of sulphate sulphur. I™ this reaction flask is 4 pour 150 cm1 recovery mixes include a hot plate 7, the power of which adjust so that the solution in the flask was boiled for 5−7 PIM and through the setting the nitrogen flow at a speed of 15−25 bubbles for 10 seconds, do Not connect the receiver, the recovery mixture is boiled for 1 h for further purification from sulfur. Then, in the receiver 6 pour absorber consisting of 10 cm3 of the polarographic background, and, by connecting the receiver to the fridge, again boiled for the recovery mixture for 30 min. the solution from the receiver is transferred into the cell and remove polarogram solution in the reference experiment. The value of the reference experiment should not exceed 0.05 mg of sulfur. Otherwise the Stripping of sulfur from the mixture again.
Then the recovery mixture was cooled and the refrigerator is connected to the receiver with the absorption solution. In a reaction flask is introduced 2 cm3 of a standard solution S sulfate sulfur, which corresponds to 2 mg of sulfur, include a hot plate 7 and distilled sulfur for 30 min. in Parallel, remove polarography without distillation polarogram the absorption of a solution containing 2 µg of sulfide sulfur. The results of determinations of sulfate and sulfide sulfur shall not differ by more than 10 &. The reason for neskhodimov results can serve as a leakage of the installation. About the tightness of the installation is judged by the coincidence of the velocity of nitrogen bubbles in the bubbler I and the receiver 6.
After the distillation of sulfur of the standard solution, again check the value of the reference experiment, and thereby verify the completeness of the distillation of sulfur from a standard solution of sulphate sulphur.
2.3. Analysis
2.3.1. In the receiver 6 pour 10 cm3 of the polarographic background and attached to the refrigerator, checked the installation. The recovery in chilled mixture is injected a portion of the antimony with a mass of 5 g brand Soooooo, weight 2 g brand Swoooop or a mass of 1 g DM Sooooo, Soooo. Include tile and distilled hydrogen sulfide 30 PIM from the moment of boiling the recovery of the mixture in the nitrogen flow. The solution from the receiver is transferred into the cell with the external anode and remove polarogram of sample solution from negative 0.4 to negative 0.8 V. the Potentials are given relative to the saturated calomel electrode. And to the refrigerator distillation unit connect the receiver with the absorption solution and determine the value of the reference experiment and completeness algonkin of sulfur from sample. Then removed the sulfide from a standard solution of sulphate sulphur with a sulphur content close to its content in the sample.
Mass fraction of sulfur in the sample is determined by comparing the wave height of the sulfide in the absorption solution post Stripping of hydrogen sulfide from a sample of tests with wave height of sulphide after Stripping of hydrogen sulfide from the standard solution.
2.4. Processing of the results
2.4.1. Mass fraction of sulphur (AO in percent is calculated by the formula
tan-Nl
X=-77−7″ — 10 4,
(I, — N3) t
where /I is the mass of sulfur in the standard solution, µg;
H — the height of the peak of the sulfide in the absorption solution after Stripping of hydrogen sulfide from a sample of a sample, mm;
/ / the height of the peak of the sulfide in the absorption solution after ugoki sulfide standard solution, mm;
N2 — the height of the peak in the reference experiment, mm; t — the weight of antimony,
2.4.2. The difference between the two results of parallel measurements and the difference between the two results
51
C. 4 GOST 1367.7−83
analysis at confidence level P — 0.95 does not exceed the allowable absolute differences of precision and reproducibility, are given in table. 1.
Table I
Loaa mass of sulfur. % Allowable absolute difference, % layer and optionally the first to produce m OS Or ti 0.000005 to 0,000010 in the key. 0,000004 0,000005 SV. 0.000010 • 0,000020 * 0,000005 0,000006 * 0.000020. 0.000050 * 0,000010 0,000012 1″ 0.00005 * 0,000010 * 0,00002 0.00003 0.00010 * 0,00020 * 0,00003 0.00004 0.00020 * 0,00050 * 0.00005 0.00006 0.0005 «0,0010 0,0001 0.0002
| Loaa mass of sulfur. % | The absolute allowable difference, % | ||
| layer and the optionally | qau produce m OS ti | ||
| Or 0.000005 to 0,000010 in the key. | 0,000004 | 0,000005 | |
| SV. 0.000010 • 0,000020 * | 0,000005 | 0,000006 | |
| * | 0.000020. 0.000050 * | 0,000010 | 0,000012 |
| 1» | 0.00005 * 0,000010 * | 0,00002 | 0.00003 |
| 0.00010 * 0,00020 * | 0,00003 | 0.00004 | |
| 0.00020 * 0,00050 * | 0.00005 | 0.00006 | |
| 0.0005 «0,0010 | 0,0001 | 0.0002 | |
3. VOLUMETRIC METHOD
The method is based on extraction of sulfur from the metal antimony with hydrochloric acid. The liberated hydrogen sulfide capture solution of a mixture of acetates of cadmium and zinc. Sulfur content is determined volumetric iodometric method.
3.1. LP parature, reagents and solutions the hot plate according to GOST 14919−83.
Water jet pump laboratory according to GOST 23932−90.
Burette for NTD with a capacity of 25 cm3.
Beakers according to GOST 1770−74 with a capacity of 1 DM5.
Volumetric flasks according to GOST 1770−74 with a capacity of 1 DM\
Flask, glass, laboratory for GOST 25336−82 with a capacity of 250 cm5.
Tube rubber cone on the other 381051835−88.
Hydrochloric acid by the GOST 3118−77, diluted 2:1, 1:1.
For acetic acid GOST 61−75.
Zinc acetate according to GOST 5823−78 and a solution with a mass fraction of 1%.
Cadmium acetate on the other 6−09−5446−89.
The absorption solution: 5 g of cadmium acetate and 20 g of zinc acetate was placed in a beaker with a capacity of 1 DM3, dissolved in water, poured 5 cm3 of acetic acid and topped up to the mark with water, mix.
Potassium iodide according to GOST 4232−74.
Potassium hydroxide according to GOST 24363−80.
Ignominously potassium (potassium Iodate) according to GOST 4202−75, 0.01 M solution: 68 g potassium iodide, 1.6 g of potassium hydroxide and a portion of the iodine that potassium weight 0.3567 g was dissolved in water, transferred to a volumetric flask with a capacity of 1 DM3, was adjusted to the mark with water and mix.
I cm3 of 0.01 mol/DM5 solution of potassium Iodate corresponds to 0,00016 g of sulfur.
The starch according to GOST 10163−76, a solution with a mass fraction of 1%.
Setup for the determination of sulfur (hell. 2).
(Changed edition, Rev. No. 1).
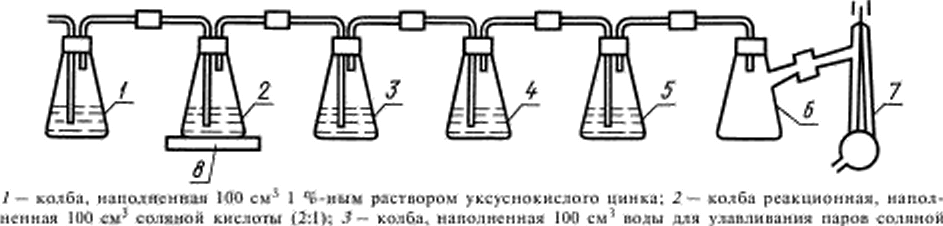 |
| Acid and arsenic: 4 — bulb, filled with 100 cm3 of an absorption solution: S — bulb control, full 60 cm' absorption of the solution: 6 — bulb Boone yuna: 7 — pump polstrany: 8 — the unit |
Damn. 2
52
GOST 1367.7−83 C. 5
3.2. Preparation for assay
3.2.1. Collect the installation of the five conical flasks, flasks, and Bunsen water jet pump. Five flasks closed with rubber stoppers with two holes for glass tubes. Glass tube, the inlet gas is lowered almost to the bottom of the flasks. The glass tube leading out the gas is cut off near the tube. Glass tube spliced vinyl chloride tube and connected to the flask Banaina and to water-jet pump. Installation gather tightly. Her integrity is judged by matching the speed of passage of air bubbles in the first and in the fifth flask when included the water-jet pump.
(Changed edition, Rev. No. 1).
3.3. Analysis
3.3.1. A portion of the antimony with a mass of 5 g brands, Soooo, MSA or a mass of I g brands SS1 and SS2 are placed in the filter paper, carefully wrapped and thrown into reaction flask 2 containing 100 cm1 hydrochloric acid (2:1). Quickly close the flask with a stopper and through the device leak air, including water-jet pump. The speed of the bubbles from the first to the fifth of the bulb should be the same and 3−4 bubbles per second. Then turn the hot plate And heat the contents of the reaction flask to a boil, reduce the heat electric switch and boiled for 10 min. Terminating the heating of the reaction flask 2, disconnect the absorption flask 4 from the device, wash with water a long tube and, after cooling the yellow emulsion of the sulphides of cadmium and zinc poured into the flask 5 cm* starch, 10 cm5 hydrochloric acid (1:1) and rapidly titrated 0,01 mol/DM1 with a solution of potassium Iodate till the solution is blue. Then pour another 5 cm' of hydrochloric acid (1:1) and, if the color of the solution disappeared, titrated further. For large sulfur contents the operation of privaie hydrochloric acid repeated 2−3 times. After the solution dotit with ethyl to a stable blue color, the absorption solution from the flask 5 is poured into the titrated solution. If the solution is colorless, then it again dotirovat steady to weak blue staining.
Absorption solution — the water in the flask 3 — it is necessary to change each time the Stripping of sulfur from a new sample.
Control experiments are performed, passing through the device in air for 10 min.
(Changed edition, Rev. No. 1).
3.4. Processing of the results
3.4.1. Mass fraction of sulfur (L) in percent is calculated by the formula
U-0.00016 — 100 ton *
where V is the volume of 0.01 mol/DM5 solution of potassium Iodate used for titration minus the average value of the two control experiments, cm3;
0.00016 — weight sulfur, corresponding to I cm* exactly 0.01 mol/DM3 solution of potassium Iodate, g: t — the weight of antimony,
3.4.2. The difference between the two results of parallel measurements and the difference of two analysis results with a confidence level P — 0.95 does not exceed the allowable absolute differences of precision and reproducibility, are given in table. 2.
Table 2
| Masson the share of SSRI, I- | The allowable absolute difference* % | |
| convergence | howling p ro n 1вод and m OST and | |
| From 0.005 to 0.010 incl. | 0.002 | 0.003 |
| SV. 0.010 «0.020 . | 0,003 | 0.004 |
| * 0.020 ► 0.050 « | 0,005 | 0.006 |
| * 0.050. 0.1 (K) « | 0.01 | 0.013 |
| * A 0.10 * 0.20 | 0,02 | 0.03 |
53







